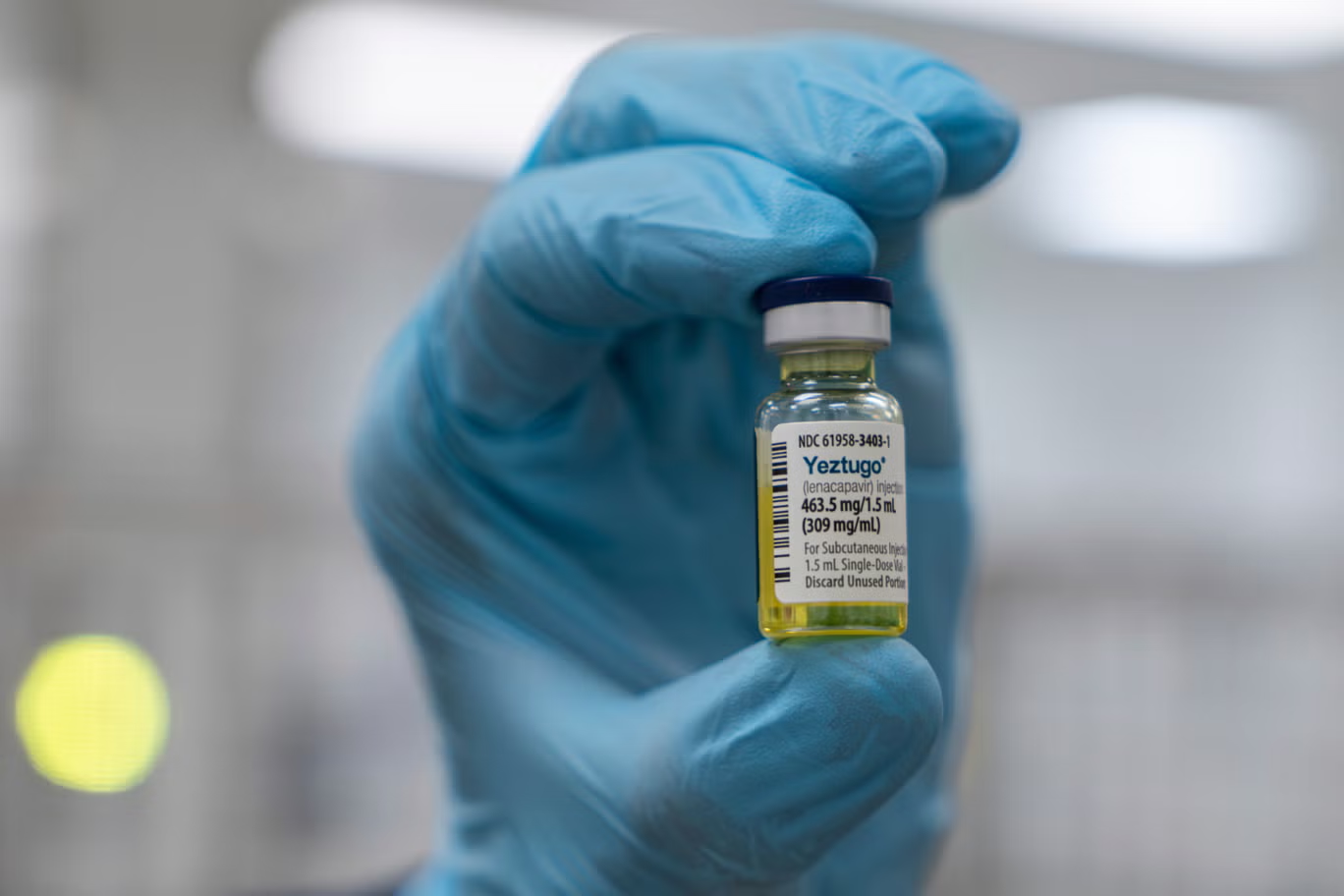
The Uganda AIDS Commission has announced plans to introduce a revolutionary HIV prevention drug, Lenacapavir, following global research confirming its effectiveness in providing 100% protection against HIV infection when administered every six months.
Latest
Museveni Questions Cattle Compensation Fraud in Teso
Mpigi LCV Chairperson, Service Commission Officials Arrested Over Job Bribery Scandal
Nakyobe Rallies Local Government Leaders on Proactive Leadership
Nambi Promises Practical Solutions as She Joins Kawempe North Race
Todwong Rallies NRM Parish Leaders to Champion Wealth Creation
Museveni: Africa’s Biggest Problem Is Underutilised Resources
Kenya Protests Are Coup Attempt’, says Minister
Court Rejects State Request for Besigye Phone Data
Gov’t Issues 30-Day Ultimatum to Car Bonds On Nakawa–Kireka Road Reserve
EC Begins Nominations for Youth, PWDs, and Older Persons Committees
Otuke Launches 16.9km Community Road to Boost Oilseed Trade
URSB Launches First-Ever Case Digest to Boost Business Confidence and Legal Clarity
In a press release issued on Saturday, the Commission revealed that Uganda was among the countries involved in the groundbreaking research that demonstrated the efficacy of Lenacapavir as a pre-exposure prophylaxis (PrEP). The long-acting injectable has already been approved by the US Food and Drug Administration (FDA) and will be rolled out in Uganda after consultations with stakeholders and compliance with national regulatory standards.
The Uganda AIDS Commission stated that the Ministry of Health, working with relevant partners, will take several key steps to facilitate the drug’s use in the country. These include:
- Evaluation and licensing of Lenacapavir by the National Drug Authority.
- Updating national HIV prevention guidelines to reflect its use.
- Training healthcare providers to prescribe and monitor the drug effectively.
- Adding Lenacapavir to the country’s essential medicines list.
- Revising data systems to enable better tracking and accountability for its usage.
Once these steps are completed, Lenacapavir will become part of Uganda’s official HIV prevention program, offering new hope to individuals at high risk of infection. In the interim, existing oral PrEP will continue to be offered at designated health facilities, while another injectable option, Cabotegravir—administered every two months—is already in use.
“This is a major leap in our fight against HIV. We remain committed to delivering innovative and effective solutions as we work to end AIDS as a public health threat by 2030,” the Commission said in the statement.
Lenacapavir’s arrival is expected to significantly improve adherence and convenience for users, reducing the burden of daily pills or frequent injections and boosting Uganda’s efforts to curb new HIV infections.